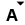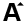Table of Gemstones Hardness, Specific Gravity, R.I.
- How to calculate Specific Gravity - Direct Weighing Method
- How to calculate Specific Gravity - Heavy Liquids
The quantity of matter in a given space is referred to as DENSITY. Expressed scientifically, it is the mass of any substance contained in unit volume. Thus the mass, or quantity, of matter contained in a cube of lead is much greater than that contained in a cube of wood of the same size. The densities of gemstones depend on the closeness with which their atoms are packed and on the weight of those atoms. A Two-carat diamond is much smaller than an amethyst (quartz) of the same weight. To say that a diamond is heavier than an amethyst conveys little information; to arrive at an exact comparison, we must compare the weight of equal volumes of each. Since it would be difficult otherwise to compare volumes accurately, this is accomplished by comparing the weight of each stone with the weight of an equal volume of water. The number of times a stone is heavier than the weight of its own volume of water is called its SPECIFIC GRAVITY (also written S.G. or Sp. Gr.). Thus the specific gravity of gemstone is the ratio of its density to the density of water.
Specific gravity is technically defined as the ratio obtained by dividing, the weight of a body by the weight of an equal volume of distilled water at a temperature of 4°C. (39.2° F.). Water at 4°C. has a maximum density and hence can be used as a standard, but in gem testing it is usually possible to obtain sufficiently accurate results without considering temperature.
The specific gravity of quartz is 2.66, which means that a quartz gem of a given size weight 2 and 66/100 times as much as a volume of water of the same size. Specific gravity is represented by a number that is definite for each species, within certain narrow limits. Hence its determination offers a sure and ready test in the identification of un-mounted fashioned gems without injuring then.
Specific gravity is an important property, not only for the purpose of identification, but because of its effect on the relative size per carat of gems. For example, a one-carat emerald is considerably large than a one-carat diamond, which, in turn, is larger than a one-carat zircon.
Stated another way, the higher the S.G., the smaller the stone per carat, and must give the stones size in millimeters rather than in weight, if he is replacing the original stone with one of another species. Many jewelers become adept at estimating the weight of a diamond by appearance; few, however, are equally proficient in estimating the weight of colored stones. To do so requires a general idea of the relative specific gravities of the various stones. As a further example, a one carat amethyst is much larger in fact, perhaps two-thirds greater in diameter than a zircon of equal weight.